Introduction:
Chemistry, with its intricate dance of molecules and compounds, unveils the mysteries of solubility, a fundamental concept governing the dissolution of substances in solutions. From the serene clarity of a saltwater lake to the bustling complexity of pharmaceutical formulations, solubility dictates the interactions that shape our world. In this in-depth exploration, we embark on a journey through the multifaceted realm of solubility, uncovering its diverse types, underlying principles, and myriad applications, complemented by vivid illustrations capturing its essence.
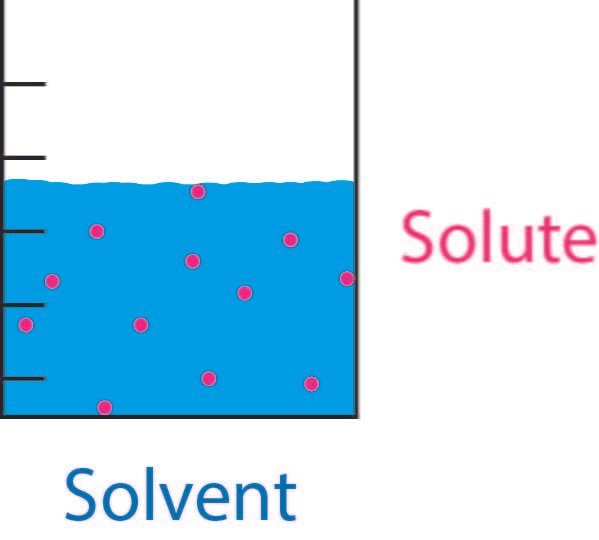
The Diverse Types of Solubility:
Solubility manifests in various forms, each characterized by distinct interactions and behaviors:
- Ionic Solubility: Ionic compounds dissolve in polar solvents through ion-dipole interactions. This type of solubility is exemplified by the dissolution of table salt (NaCl) in water, where water molecules surround and solvate the individual ions.
- Molecular Solubility: Molecular solutes, such as sugar (sucrose) and ethanol, dissolve in solvents via intermolecular forces like hydrogen bonding and dipole-dipole interactions. The dissolution of sugar in water illustrates this type of solubility, where water molecules form hydrogen bonds with sugar molecules, facilitating their dispersion.
- Gas Solubility: Gases dissolve in liquids through mechanisms governed by Henry’s law, where the solubility of a gas is directly proportional to its partial pressure above the liquid. Carbon dioxide dissolving in soda water serves as a classic example of gas solubility.
- Solid Solubility: Solids can dissolve in other solids to form solid solutions, where atoms, ions, or molecules of the solute are uniformly dispersed within the solvent. An example is the alloying of metals like brass, which is a solid solution of copper and zinc.
Applications Across Chemistry:
Solubility finds diverse applications across various fields, driving innovations and discoveries:
- Pharmaceutical Formulation: Understanding solubility is critical in drug development, influencing factors such as drug absorption, bioavailability, and formulation stability. Techniques like solid dispersion are employed to enhance the solubility of poorly soluble drugs, improving their therapeutic efficacy.
- Environmental Remediation: Solubility governs the transport and fate of pollutants in environmental systems. Remediation strategies leverage solubility principles to design efficient processes for the removal and treatment of contaminants in soil and water.
- Food and Beverage Industry: Solubility impacts the sensory attributes and stability of food and beverage products. Adjusting solubility parameters allows manufacturers to optimize formulations, ensuring desirable texture, flavor, and shelf life.
- Material Synthesis: Solubility plays a pivotal role in the synthesis and processing of materials such as polymers, nanoparticles, and composites. Controlling solubility enables the fabrication of materials with tailored properties and functionalities for diverse applications.
Challenges and Advances in Solubility Research:
Despite its significance, solubility presents challenges and opportunities for advancement:
- Predictive Modeling: Accurate prediction of solubility remains a complex endeavor due to the interplay of multiple factors. Computational methods and machine learning techniques are being developed to enhance predictive models, aiding in drug design and environmental assessment.
- Green Solvent Design: With increasing environmental awareness, there’s a growing emphasis on designing sustainable solvents with minimal ecological footprint. Green chemistry principles guide the development of solvents that are non-toxic, renewable, and biodegradable.
- Supramolecular Approaches: Harnessing supramolecular interactions offers novel strategies for controlling solubility and designing functional materials with tailored properties. Self-assembled structures and host-guest complexes hold promise for applications ranging from drug delivery to catalysis.
Conclusion:
Solubility, in its myriad forms, pervades every aspect of chemistry, from the microscopic realm of molecular interactions to the macroscopic landscape of industrial processes. As our understanding deepens and technology advances, the exploration of solubility continues to unveil new frontiers and possibilities, shaping the trajectory of scientific inquiry and innovation. In this intricate dance of dissolution, the principles of solubility serve as a guiding light, illuminating pathways towards a more sustainable and interconnected world.
Leave a Reply