Chemical Reactions:
A chemical reaction is a process in which one or more substances (reactants) are converted into one or more different substances (products) with new chemical and physical properties. Chemical reactions involve the breaking of chemical bonds in the reactants and the formation of new bonds to produce the products.
Chemical Kinetics:
Chemical kinetics is the study of the rates of chemical reactions and the factors that influence reaction rates. Key concepts in chemical kinetics include reaction rates, rate laws, reaction mechanisms, activation energy, and reaction order.
Factors Affecting Reaction Rates:
- Nature of Reactants: Different substances react at different rates depending on their chemical properties.
- Concentration: Generally, higher concentrations of reactants lead to faster reaction rates because there are more reactant molecules colliding with each other.
- Temperature: Increasing temperature usually increases reaction rates because it provides more energy to reactant molecules, leading to more frequent and energetic collisions.
- Catalysts: Catalysts are substances that speed up reactions by providing an alternative reaction pathway with lower activation energy, allowing reactions to occur more readily.
- Surface Area: In reactions involving solids, increasing the surface area of the solid reactant can accelerate the reaction rate because it exposes more reactant particles to the other reactants.
Types of Chemical Reactions:
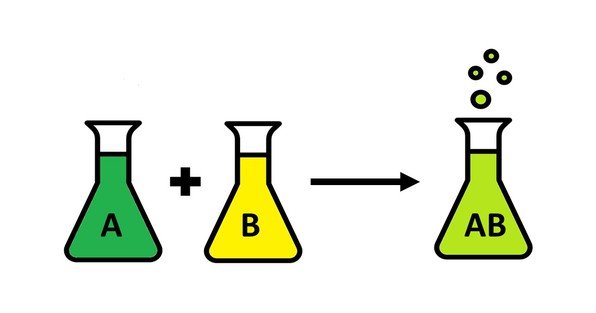
- Combination (Synthesis) Reactions:
[A + B \rightarrow AB]
Example: (2H_2 + O_2 \rightarrow 2H_2O) - Decomposition Reactions:
[AB \rightarrow A + B]
Example: (2H_2O \rightarrow 2H_2 + O_2) - Single Replacement (Displacement) Reactions:
[A + BC \rightarrow AC + B]
Example: (Zn + 2HCl \rightarrow ZnCl_2 + H_2) - Double Replacement (Metathesis) Reactions:
[AB + CD \rightarrow AD + CB]
Example: (NaCl + AgNO_3 \rightarrow NaNO_3 + AgCl) - Combustion Reactions:
Combustion reactions involve a substance reacting with oxygen gas to produce carbon dioxide and water.
Example: (C_6H_{12}O_6 + 6O_2 \rightarrow 6CO_2 + 6H_2O)
Rate Laws and Rate Equations:
The rate of a chemical reaction is often expressed using a rate law equation, which relates the rate of reaction to the concentrations of the reactants. The general form of a rate law for a reaction involving reactants A and B is:
[Rate = k[A]^m[B]^n]
where:
- (k) is the rate constant,
- (m) and (n) are the reaction orders with respect to reactants A and B, respectively.
Example of Rate Law:
Consider the following reaction:
[2NO_2(g) \rightarrow 2NO(g) + O_2(g)]
The rate law for this reaction might be:
[Rate = k[NO_2]^2]
This indicates that the rate of the reaction is directly proportional to the square of the concentration of (NO_2).
Reaction Mechanisms:
Reaction mechanisms describe the sequence of elementary steps by which reactants are converted into products. These steps involve the breaking and formation of chemical bonds and often include intermediate species. A complex reaction may involve multiple elementary steps, and the overall rate of the reaction is determined by the slowest step, known as the rate-determining step.
Summary:
Chemical reactions and their kinetics are essential for understanding how substances transform into new substances over time. Factors such as concentration, temperature, catalysts, and reaction mechanisms influence reaction rates. Different types of reactions, including combination, decomposition, single replacement, double replacement, and combustion, illustrate various ways in which substances can react with each other. Rate laws and reaction mechanisms provide insight into the quantitative and qualitative aspects of chemical reactions.
Leave a Reply